X-rays shed light on how anti-asthmatic drugs work
A new study uncovers how a critical protein binds to drugs used to treat asthma and other inflammatory diseases.
By Jennifer Huber
By studying the crystal structure of an important protein when it was bound to two drugs widely prescribed to treat asthma, an international team of scientists has discovered unique binding and signaling mechanisms that could lead to the development of more effective treatments for asthma and other inflammatory diseases.
The protein, called cysteinyl leukotriene receptor type 1 (CysLT1R), controls the dilation and inflammation of bronchial tubes in the lungs. It is therefore one of the primary targets for anti-asthma drugs, including the two drugs studied: zafirlukast, which acts on inflammatory cells in the lungs, and pranlukast, which reduces bronchospasms due to allergic reactions.
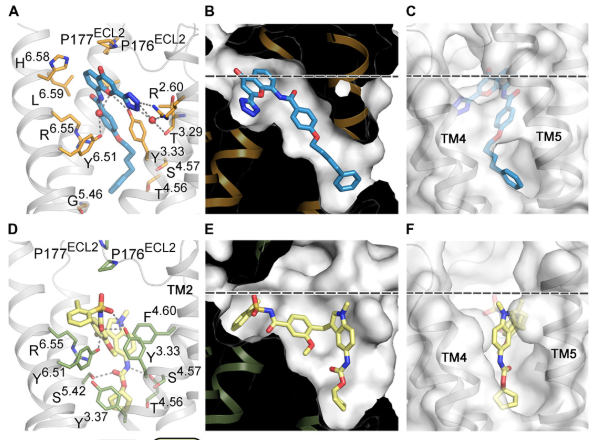
Using the Linac Coherent Light Source (LCLS) X-ray free-electron laser at the Department of Energy’s SLAC National Accelerator Laboratory, the team bombarded tiny crystals of CysLT1R-zafirlukast with X-ray pulses and measured its structure. They also used X-rays from the European Synchrotron Radiation Facility in Grenoble, France to collect data about CysLT1R-pran crystals. They published their findings in October in Science Advances.
The researchers gained a new understanding of how CysLT1R interacts with these anti-asthma drugs, observing surprising structural features and a new activation mechanism. For example, the study revealed major differences between how the two drugs attached to the binding site of the protein. In comparison to pranlukast, the zafirlukast molecule jammed open the entrance gate of CysLT1R’s binding site into a much wider configuration. This improved understanding of the protein suggests a new rationale for designing more effective anti-asthma drugs.
The study was performed by a collaboration of researchers at SLAC; Moscow Institute of Physics and Technology, Russia; University de Sherbrooke, Canada; University of Southern California; Research Center Juelich, Germany; Universite Grenoble Alpes-CEA-CNRS, France; Czech Academy of Sciences, Czech Republic; and Arizona State University. Crystallography data collection of CysLT1R-pran crystals was performed at the European Synchrotron Radiation Facility in Grenoble, France.
Citation: Aleksandra Luginina et al., Science Advances, 09 October 2019 (10.1126/sciadv.aax2518).
Contact
For questions or comments, contact the SLAC Office of Communications at communications@slac.stanford.edu.
SLAC is a vibrant multiprogram laboratory that explores how the universe works at the biggest, smallest and fastest scales and invents powerful tools used by scientists around the globe. With research spanning particle physics, astrophysics and cosmology, materials, chemistry, bio- and energy sciences and scientific computing, we help solve real-world problems and advance the interests of the nation.
SLAC is operated by Stanford University for the U.S. Department of Energy’s Office of Science. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit energy.gov/science.