Research Zooms in on Enzyme That Repairs DNA Damage from UV Rays
Biochemical 'action shots' with SLAC’s X-ray laser could help scientists develop synthetic enzymes for medicine and answer fundamental questions about how enzymes change during chemical reactions.
By Miyuki Dougherty
A research team at the Department of Energy’s SLAC National Accelerator Laboratory is using the Linac Coherent Light Source (LCLS) to study an enzyme found in plants, bacteria and some animals that repairs DNA damage caused by the sun’s ultraviolet (UV) light rays.
By studying this enzyme, called DNA photolyase, with the ultrabright and ultrafast pulses of the LCLS X-ray laser, researchers finally have the opportunity to watch the enzyme in action as it catalyzes a chemical reaction in real time and at the atomic scale to resolve longstanding debates about how these enzymes work. Ultimately, this knowledge could be used to engineer improved synthetic versions of enzymes that drive crucial reactions in biological systems, or to produce novel enzymes that do not exist in nature.
“The biochemical reactions performed by enzymes are at the heart of the adaptability and efficiency of living things,” says Thomas Joseph Lane, an associate staff scientist at LCLS. “But the details of how enzymes work is hidden in chemical processes that occur on extremely short timescales, down to millionths of a billionth of a second, so we needed LCLS to reveal their secrets.”
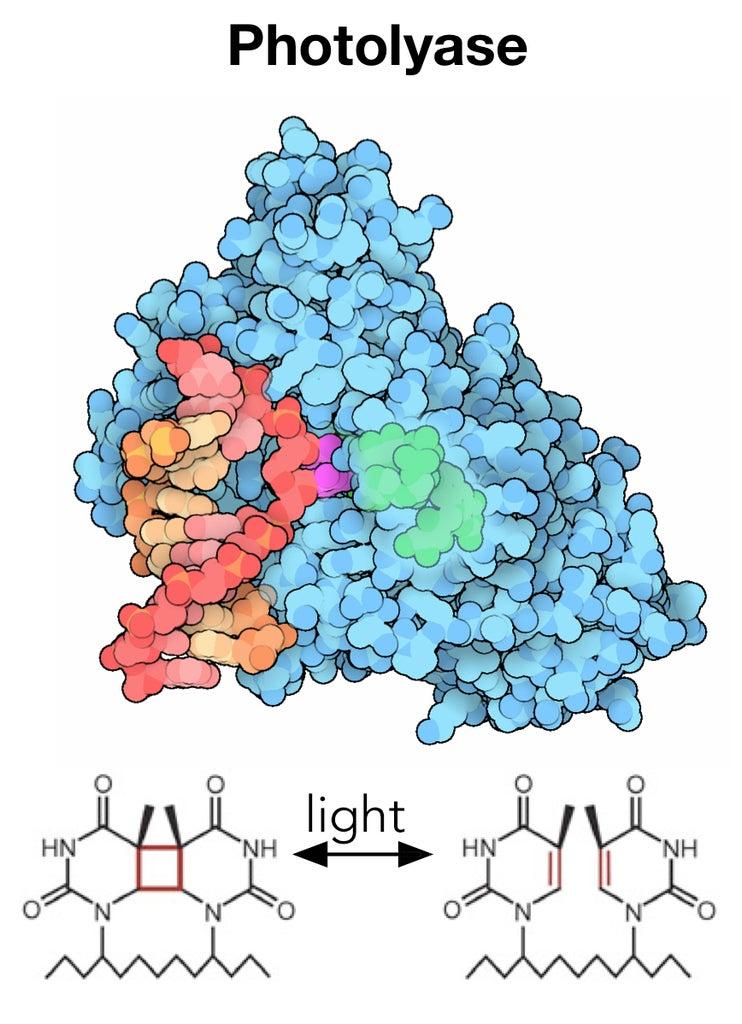
A Powerful Repair Machine
In just a few seconds, ultraviolet light from the sun can damage DNA by creating hundreds of unwanted links within DNA’s double helix. These modifications make the genetic material bulky and unreadable by DNA replication tools, leading to permanent mutations that can cause cancer and other diseases if left unrepaired.
But the same sunlight that carries damaging UV rays also contains blue light that can induce photolyase to quickly repair any DNA damage.
Photolyase is thought to be one reason why plants – which have hours of exposure to the sun each day – are less susceptible to UV damage than humans, who lack photolyase. Humans and other mammals must fall back on alternative DNA repair mechanisms (or avoid going out into the sun altogether).
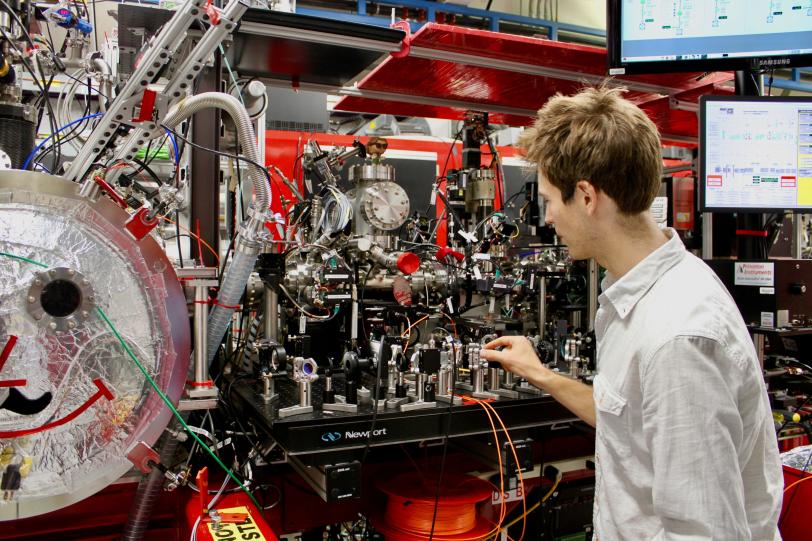
Using an Ultrafast X-ray Camera
With LCLS, researchers now have access to some of the fastest and brightest X-ray laser pulses in the world to study how living things defend themselves from UV damage.
Earlier this year, for instance, a team of scientists led by Thomas Wolf, an associate staff scientist at SLAC, used LCLS to see the first step of a protective process that prevents UV damage in the DNA building block thymine.
“Before LCLS, other X-ray ‘cameras’ were too slow,” Lane explains. “Trying to precisely image enzymes and other proteins with those X-ray sources would be like trying to take an action shot of Michael Phelps swimming with an old camera. You would only get a few blurry images over his entire 100-yard butterfly event, which would hardly make for an exciting or informative photo.”
But with LCLS, he says, “Imagine a series of high-resolution shots in sequence – you would be able to capture every drop of water and every twist of Phelps’ wrist as he butterflies. That’s what LCLS lets us do when visualizing enzyme activity.”
Building Better Enzymes
In contrast to Wolf’s experiment on how DNA protects itself from damage, Lane’s team is studying how photolyase repairs UV damage once protective mechanisms have failed. Photolyase can be controlled with great precision by exposing it to light, making it an ideal enzyme to study using laser-generated light.
To see photolyase chemistry in detail, the researchers activated the enzyme with a carefully controlled light pulse from a laser. They subsequently exposed the enzyme to the LCLS-generated X-ray pulse, creating a characteristic X-ray scatter pattern in a specialized detector. The analysis of scattered X-ray data revealed chemical and structural changes in the enzyme at atomic level and occurring at a time scale of a millionth of a billionth of a second.
One of the ultimate goals of studying the enzymatic DNA repair process is to engineer synthetic enzymes that mimic but are even better than those found in nature.
“There are still some major gaps in our understanding of how enzymes work, highlighted by the fact that man-made enzymes have yet to match nature’s performance,” says Lane. “We hope our experiments here at LCLS will help us bridge those gaps, getting us closer to understanding and harnessing the chemistry living things do every day.”
The research team studying the photolyase repair mechanism includes scientists at SLAC, the Center for Free-Electron Laser Science (CFEL) in Germany, Chapman University, KTH Royal Institute of Technology in Stockholm, Sweden, the Ohio State University, Stanford University, and the University of Gothenburg, Sweden.
LCLS is a DOE Office of Science user facility.
For questions or comments, contact the SLAC Office of Communications at communications@slac.stanford.edu.
SLAC is a multi-program laboratory exploring frontier questions in photon science, astrophysics, particle physics and accelerator research. Located in Menlo Park, Calif., SLAC is operated by Stanford University for the U.S. Department of Energy's Office of Science.
SLAC National Accelerator Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.