so good evening and welcome to the next installment of the SLAC public lectures So today we're very lucky to
have Liz Ryland from the pulse Institute here at slack and Stanford to talk to us
uh Liz is from Louisiana originally did her ba at Louisiana or BS I guess at
Louisiana Tech um then from there she got to the University of Illinois one of
the the big places actually for condensed matter physics um she apparently built her own little
x-ray laser and did so well that she got to come here and work with the big one that we have here at slack and so she's
been a post to at the pulse Institute um she's going to talk to us about uh
looking for trolls under the electron Bridge so we'll find out what that means
good luck all right thanks for the introduction Michael so as Michael said
we're going to search for trolls under an electron Bridge today and we're going to spend a while figuring out what on Earth I'm talking about so when I gave
this practice talk they wanted more info about me so I am indeed from Louisiana uh yeah it's a great place
it's the land of Marty gr food and like aggressively hot sunlight and a lot of plants that grow from that and that's
really all that I'm about I'm from sh for so the northern part of the state not exactly New Orleans which is way
down there but I have family in Lafia kind of capital of Cajun Country and kind of have been all over the state and
really there is sunlight everywhere sunlight and plants grow big and strong and the older I get the more impressed I
got by how massive plants can grow and how much they can do deriving energy
just from sunlight and Soil and Water so as Michael said I
after like childhood in high school and stuff I went to Louisiana Tech University it's a big engineering school
in Louisiana and I got my degree in chemistry and I worked at a college radio station and from doing those two
things I realized that chemistry is cool and with my undergraduate degree I really just only had got the bearest
foundation so I really wanted to know more and so I applied to graduate school and I decided to go to the University of Illinois at Urbana-Champaign as Michael
said it's a very big school and there I joined the Josh verise group where we built this tabletop XUV instrument which
instead of making really high energy x-rays in about a mile we made really
soft energy X-rays and about six feet inside our own lab and we got to kind of build that all from scratch it was
really cool and I used that to really start studying molecules on a fundamental level really tracking the
electron movement at the speed that they actually move at from there I went to the US Naval Research lab for a post to I
kind of expanded into IR spectroscopy and that's where I spent the Lost plague year of 2020 and then from there I moved
on to slack uh you know Co not a great time for anyone so I moved on to Slack and
even though I had been working in IR spectroscopy I wanted to expand my horizons I really like X-rays and all of
the different things you can do when you're using those as your primary instrument so at slack I joined uh Kelly
Gaffney and Amir cardones Han in the solution phase chemistry group of the Stanford pulse Institute of the slack
National accelerator lab really lots of titles what that means is that we are based at slack and we're really an
enduser for these giant instruments so we're not part of making the XR but we're part of using them to answer
fundamental scientific questions so getting into the bulk of the talk why we even want to study
photochemistry is it because there's a massive ball of energy in the sky that's glowing on us constantly pretty much so
the sun is really powerful so the sun is really powerful
it's constantly doing Fusion so it's taking two hydrogen smashing them into a helium atom and releasing tons and tons
of energy as I have up there it fuses over 600 million tons of hydrogen per
second and releases massive amounts of energy almost four time 10 26 watts per
day that's almost a trillion times a trillion times a trillion of watts of energy compared to what actually reaches
Earth because that's emitting an all you know directions from this sphere in the sky it's Crossing 93 million miles to
get to the Earth's surface and at the Earth's surface you're going to absorb almost 200,000 terawatts of energy just
distributed hitting every single continent things like that so really tons and tons of energy to put this in
context that is 10,000 times the global energy use
today we all know that the sun is powerful in California we use it to grow
nutrients inside plants and create harvests and fruits and vegetables and weeds use them to grow aggressively and
no matter what we can do we can't actually get rid of weeds because they're powered by the sun which is incredibly
powerful so we have been trying as chemist to figure out how can we utilize
the sun's energy for a long time starting with uh gako chiman an Italian
photo-chemist from the late 1800s he was Nobel nominated for the Nobel prize
Nobel Prize different times and he's thought to be the father of photochemistry so he did it the first
photochemistry experiment in 1886 he pretty much exposed things to
sunlight and was like this is wild there is going to be sometimes bubbling gasses evolving in situation there's color
changes anyone who's left a jacket they really like in their car over a hot summer has come back to find their jacket half a different color this is
because the sun it sends you this this light and is going to interact with materials at a fundamental level so Tron
he started the Department of Chemistry at the University of bologna and this is a picture of him on top of the roof
of the University just with a bunch of different folks all sitting out just seeing what chemistry is going to happen
it's a great picture if you zoom in really far he actually has two different buildings covered like he's like I don't
need a lab space guys there's a roof and I have these flasks and up in the left is kind of an example of the type of
reaction so basically you have molecule you expose to sunlight and suddenly the molecules connect together almost like a
polymerization and more than that he's also thought of as the father of the idea of green chemistry so after the
turn of the century in around 1908 to 1912 he started writing these papers being like the sun is amazing guys why are
we using fossil fuels and in a seminal paper from 1912 published in science and then presented at International
conferences he pretty much announced we need to stop using fossil fuels I have this great vision of the future we don't
need to keep burning coals and polluting our environment we can convert to purely solar he had a very fun idea he didn't
quite think of solar panels instead he thought of giant glass tubes that kind of stretched out over Europe and all of
the Cities winding around and all of them were full with some gas absorbing light and for Sun and essentially just netting that straight into the
electrical grid so we didn't quite come up with his vision but we're still using the sun
today to power a lot of different sources there's three different ways we primarily Harvest energy from the Sun
there's going to be thermal energy which it gets really hot outs sometimes asphalt gets so hot you can cook eggs on
it that's basically doing chemistry so we have different ways we can Harvest this energy it's like contributes to uh
heating of the Earth's surface and you know we're all familiar with being warm in the recent years we started being
able to harvest electrical energy from sunlight solar panels so in California
alone in a year we generate around 20,000 Million per year that's around 15%
of total California energy consumption but that's not really what I'm talk today many of you are probably
familiar with those areas what instead I'm going to talk about is how can we use the sun to directly do chemical
reactions just like chimen was trying to do so there's two different main R we're
going to go for you're going to store energy in chemical bonds many of us are familiar with that how plants work
plants are going to take sunlight and use it to power the mechanism of converting water and CO2 into glucose
and oxygen which we need to breathe but glucose is very powerful and they use it to power all of these cellular functions
the other option is that you use that energy immediately or directly to power a chemical reaction an example we're all
familiar with is color change sunglasses all that is is sunlight comes in and you're making a molecular Bond or twist
or change in some way and that's going to have a different interaction with light so the way that we as a society
think we're going to imitate what plants are doing is instead of trying to make glucose and oxygen which is a very
complicated process using photosystem too a lot of great studies going on at slack about that but is this idea of
storing energy in chemical bonds so specifically hydrogen gas so that
instead of putting gasoline into your heart you'll put condensed hydrogen and then use those chemical bonds to power
uh your car so comparing hydrogen to gas
it's going to one kilogram of hydrogen is about a coil one gallon of gasoline a gallon gas will get you 25 miles and
will release a bunch of hydrocarbons into the atmosphere whereas one kilogram of condensed hydrogen is going to be
able to power your car 60 miles and more importantly when it combust hydrogen combusting with oxygen is going to have
a main output of water which is going to be better to put into the environment than any sort of
pollutant so we're already working on a bunch of different ways to make hydrogen mostly with electrolytic uh water
splitting you send in water you electricity hopefully green electricity and you're going to generate hygiene and
be able to release oxygen into the atmosphere the photo chemists dream is to just take out the middleman why are we
using this electricity that we had to generate and store and use batteries and put it into our grid why not make the
sunlight do it directly and that idea is is essentially taking water exposing it to sunlight and a catalyst and
generating hydrogen and oxygen so the question of course for a
chemist is how are we going to design a catalyst that harvests sunlights and then takes those electrons that it's
taking directly from the sunlight to do the specific chemistry needed to turn water into hydrogen so that's going to
be again breaking the bonds in water and then putting that energy and storing it into hydrogen that then breaks releases
energy and you can power a lot of things just like gasoline so how can we make this
Catalyst be powered by sunlight so a catalyst is let's call it just a molecule that does some specific chemist
like Mak hydrogen or really anything else chemistry can do a lot of stuff the idea is that when you expose it to
sunlight you're taking a ground state and you're act catalytically active so instead having to heat it up or
electrocute it or expose it to energy you ground state of some molecule this is an example of a common photocatalyst
has a ruum surrounded by some other stuff you expose it to light it moves it up an energy level into this excited
state which in essentially is taking an electron off of the ruum atom the metal
and moving it onto one of these outer sphere lians and then that electron is accessible to do some kind of chemistry
and that's great that's all well and good there's a bunch of great research on this there's actually a slack public lecture covering this topic from 2017 by
chrisan cunis talking about catching light is I believe the name of but really talking about this process so
today I'm going to talk about what do you do when that's not the chemistry you want to do that molecule can't do it you
instead want to use this it's a platinum Catalyst really commonly used in a lot of industrial processes but it has a
different system instead of from the metal to the LI you want your Li so your chlorine kind of around it to give an
electron to your metal so you shine some light on it and your light isn't quite enough doesn't work nothing happens you
don't reach the six side State this is because light as many of you know comes in a spectrum all
different colors of light are coming down from the Sun the yellow line is what hits in sunlight on top of the
atmosphere remember to the left is going to be shorter wavelength so higher
energy things like purple and x-rays eventually and then to the right is going to be your infrared light going
into microwaves and much longer wavelength and lower energy the M from here you can easily see the majority of
sunlight hitting their surface is going to be of this visible band that's actually why we see in the visible it's
the most important photons for our eyes to be able to see and this is at the top of the
atmosphere you go through the atmosphere and all the different substances absorb these specific bands of light so you
reduce the yellow into that red line so that's the radiation left over at sea level so a little bit weaker still
clearly dominated by the visible region and you can see notches in it that are going to be the specific absorption
bands of water and CO2 and other things in our atmosphere and the goal of this
is that you want to design some kind of catalyst A system that absorbs specifically in that visible region to
harvest as much of this sunlight as possible so for that system you can
actually separate it out and have a part that is going to absorb energy from the Sun and turn it into an electron and you
have a part that's going to do the specific chemistry rather than having one system that do you just connect them
you connect them using an electron bridge and that's really going to be the theme of today it's how do we make an
electron effectively move from donor the thing that is absorbing sunlight and
creating an electron to your electron acceptor which is going to be the thing that does some kind of chemistry this is
an example of what the molecule looks like in real space so going from one end to the
other however you have a competing pathway once your electron leaves your
electron doesn't really want to stay left it has all this energy it got up in the morning and was like I'm going to go on a trip but sometimes it gets there or
goes out and it loses that energy and so it's going to return home and so our entire job is kemus is trying to make a
unidirectional bridge where you have the electron go really fast and not turn
around and come back kind of similar to I'm sure one of someone in here has driven over one of these the wrong way I know that's what that laughter was so
you want uh they're called Road spikes where drive over one way the other way they puncture your tires we're trying to
design molecular Road spikes so the electron moves one way not the other and
you really want to do this because you want it to remain active as long as possible this is going to be at least
tens to hundreds of nanoseconds that's how long it takes for this molecule to run into other molecules and actually do
some chemistry this is okay though because the electron moves fast enough and we we
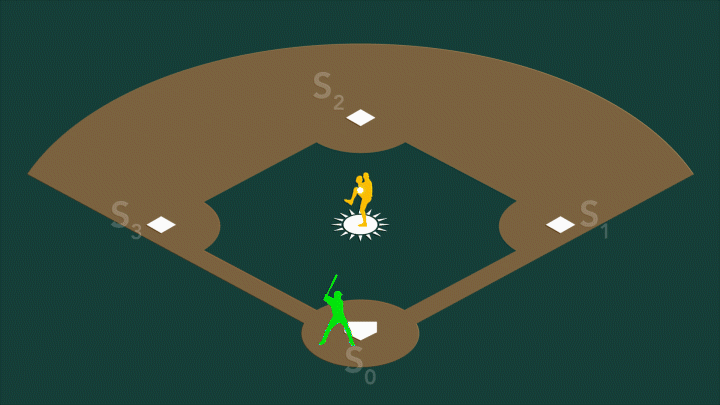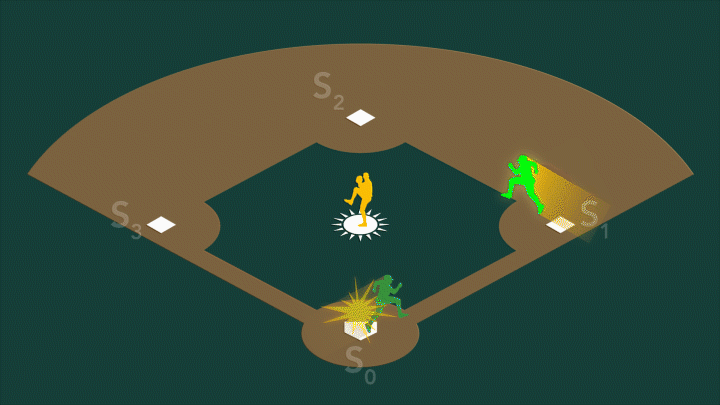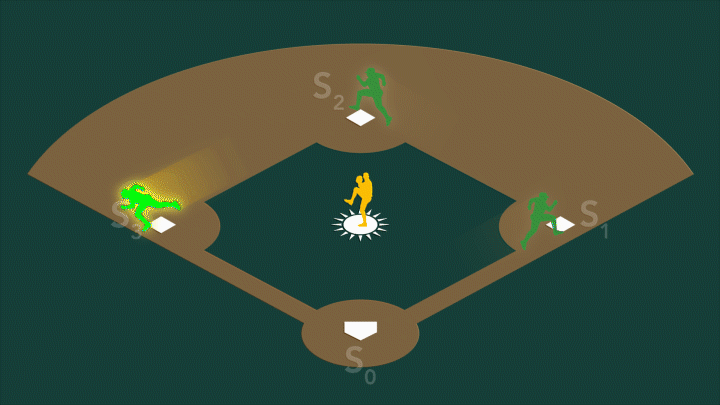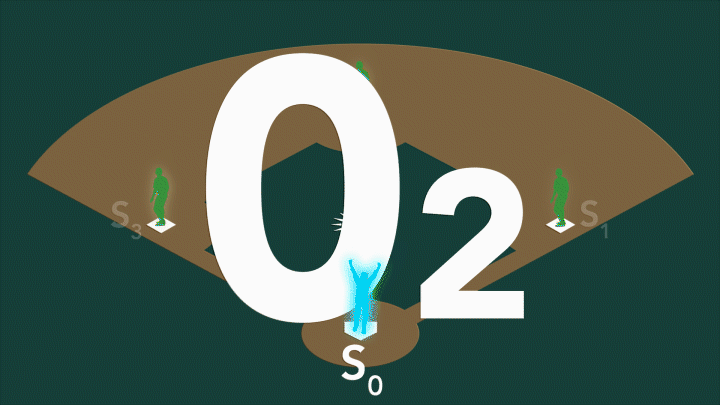
can do this so here's a slight less intimidating drawing of a molecule where you have the ranium portion is going to
generate electron and we're going to move across to the Platinum so upon photo excitation let's just kind
of watch what the electron does so you have an electron that's just hanging out in its Atomic home inside the ranium you
photo electron moves the closest part of the bridge and then a little bit of time passes it moves to the center a little
bit of time passes it moves SP a little and it finally arrives at the platinum
and we're ready to do some targeted chemistry this entire distance is going to be around 2 nanometers for comparison
the average width of human hair is around 50,000 nanometers so this is an incredibly incredibly small distance
that this electron is moving and the electron is moving incredibly fast it's going to be much much faster than a nanc
faster than a Pico thousandth of a nanc frequently on the orders of ftoc which
is a thousandth of a thousand of a nanc and then again needs to stay there
for 100 NS do that chemistry unidirectional the problem however is sometimes your electron never arrives
your collaborator Spends months making these beautiful molecules all of this theoretical design it's going to work
great it doesn't do any chemistry you look at it and the electron never arrives the question has a troll
consumed my electron and if so where has it been
several trolls how has it done this to me this is really the job of a physical
chemist which is what I identify as so there's many different kinds of chemistry there's people who do
calculations and design things there's people who stand in a lab a wet lab and do some amazing magic with vases not
vases and glasses to synthesize these molecules and then you have physical chemists who are kind of afraid of real
chemicals but are good at math and good at like using wrenches and so we build these really insane three mile long
instruments and convince everyone use them and we have a lot of fun really our goal is to figure are we're figuring out
how the molecule works and more importantly how it doesn't work and then we work with our collaborators to tell
them this is exactly where this molecule went wrong and so you can work together so they can do de novo design and fix
this Catalyst to you know make a brighter future so what do I mean by a
troll this can be the electron is stuck on the bridge your electron leaves home gets to the bridge and the bridge is
really nice why does it want to go all the way to the Platinum the bridge is really nice it feels sa stable and safe there it would be uphill in energy to go
onto the Platinum your option can also be that the bridge is too long and that there's a lot of traffic so the electron
it's going really slow and you know it would take longer to get away than it would to just turn around and go back
home that's where you have that competing back electron transfer problem and where we want to invent this idea of
like no don't go home it's very hard to go home and the last option that I'm going to talk about is maybe the
electron just gives its energy to Sol it goes on the bridge and then it meets like a really nice hobo and it gives all
its money to the hobo and the hobo like swims away into the sunset I don't I don't know how that would go anyway so
really my job is identifying how has the electron been eaten or been sent back home because it's afraid of a
troll so how do you do that how do you put cameras on an electron bridge this is really tiny this is only a few
nanometers long how are we going to put traffic cameras on a molecular Bridge
the answer is lasers because lasers are super fun all right so a quick rehash on
lasers lasers are light waves particle wave Duality they oscillate In Waves
they're very targeted and collinear so they can be powerful blue light is made of shorter waves than red light red
light is long longer waves but particle wave Duality lasers are also particles
you're delivering literal units of light energy a piece of blue light has more
energy than a pie piece of red light particle wave Duality they happen as both and that's incredibly important
for understanding how to manipulate lasers and use them to study things we can thank this kind of concept to plun
who around 1900 was like Hey things can exist in Quant of energy photons probably exist and then our favorite
dude Einstein who literally every era of science you just lead back to him somehow in 1917 he pretty much trolled
everyone and said hey guys I did some math you can definitely make laser you can build up this light and deliver it
in a coherent source so that you can shine a laser pointer woo laser pointer and have it go really long distance
without ever like losing sorry fumbled that they go in a straight line for a
really long time which means you can point them at things they aren't diffuse and I say he trolled people because he
said you could do this in 1917 and he clearly wrote out the math to do it and then it took 45 years for someone to
actually be able to physically make it the first laser that was made is a ruby laser and so imagine you have a brick of
Ruby literally we use crystals all the time Sapphire is one of the most commonly ones we use so a laser is just
any sort of gain medium coupled into an optical cavity the gain medium you shine
light on it in the Ruby laser case it was a flash lamp and by Shining Light on it you're giving a bunch of energy to
this system and everything's going to just start trying to get rid of that energy as a characteristic wavelength you
make it into a laser by putting mirrors on each end of the cavity and only something that matches like a multiple
of that cavity is going to oscillate this is why they are actually able to make a merer first so that is
microwave laser because you can imagine that making a cavity that's uh multiple
units of centimeters is much easier than one that's multiple units of nanometers and so just aligning and creating was
really hard then there was the the wild west of laser inventions in the 1960s there was
so much going on and there is actually was like a 30-year litigation on who really deserve the Nobel Prize and who
actually gets the patent for making lasers really cool stuff invented a bunch of things then came Donna
Strickland who in 1985 she made a came up with something called chirp pulse amplification which
essentially a way to make a very high power pulse laser and that enabled using
these to study chemical methods so Donna Strickland got the 2018 Nobel Prize for
this method and then the 1999 Nobel Prize went to this man named Ahmed zael
who is essentially the father of fto chemistry he's the one who took these lasers and used them to solve chemical
problems the the way you did this is that these
laser pointers that everyone's mostly familiar with these are called continuous wave lasers so they are constant L emitting in time however by
doing some interesting Optical cavity stuff you can have the lasers deliver instead
tiny at once and these pulses can be incredibly short and I'm talking microsc
and nanc followed in the 90s by PC lasers that you can control well and then after that ftoc lasers so a ftoc is
a millionth of a billionth of a second that's very very short and if you have
ftoc laser it's like a camera so you have a camera that has a very stop Fast
Flash speed so at a public lecture a few months ago someone use the example of a hummingbird with a really slow camera so
a long exposure time you can't see the hummingbird's wings but if you make your exposure time shorter and shorter and
shorter you can start resolving and seeing those hummingbird wings and that's why ftocs are really important
for studying chemistry because that is the rate at which electrons move along a bridge out of seconds even shorter than
that very great a lot of cool talks on that for interesting physics things and
then y'all can go and watch their lectures if you want to hear about them because I'm really only talking ftoc
so talking explicitly how you do this is what zel did is he was like okay one
laser is done what if we use two it's a technique called pump probe spectroscopy you use two lasers the first one
delivers a pump pulse that is going to be a piece of visible light it's going to imitate the Sun you give it to a
molecule and it goes into the excited state what happens to it if it gets exposed to light and then you come in
with a probe pulse and that again that's your camera you're taking a picture and by
changing the or not the exposure time but when you take the picture it's like if you take a picture one second two
seconds three seconds things like that and the great things about lasers is they're columnated and all light travels
at the same speed in a vacuum so by literally changing the distance that you are having on your alignment path you
change the arrival time between the two and you're taking photos throughout time
and you can really remap electron movement process so whales work all the light was
visible and we're at SLAC National Lab why am I talking about all this Optical stuff so energy describes uh the color
of the light so visible light we're now talking in EV is a unit of energy
visible light is going to be very short it's going to be like one or two EV x-rays are much much bigger they're
going to be a thousand EV or 100,000 EV the reason that uh this matters is
because this is going to be characteristic on an element by element
basis so we use slack because slack is an x-ray laser we can use tabletop
things to make Optical pulses of light but you really need these massive facilities to make incredibly short
pulses of X-ray light because they move completely different from other light and you need the source needs to be
really long because you want it to be really intense the more intense your pulse the better your resolution of your
camera so ex really short why do we use x-rays here you have a nucleus you have
a bunch of electrons sitting around your X-ray light comes in and it kicks out one of the electrons in an
inner shell and it moves it into the veilance level by absorbing that piece of light so if you have nitrogen it's a
very small distance just a little hop for that electron from home to the
veence Shell if you have a bigger atom like a metal like this ruum you have a lot more
layers of electrons and it's going to be a bigger distance that it has to go same
thing for platinum even bigger than renum so your electron has further to travel to get to to the veilance bander
to where it wants to go so a little bit of audience participation how much gas
does it take to go from the surface I'll give you an example nitrogen is 400 EV
who Brave and wants to guess what it is for
ranium no kids today adults are not
brave 1 1600 that is a good
guess so this is a huge difference and that is not because it is just a
distance change between these atoms as you get bigger and bigger the core of these atoms is pulling its electrons
even stronger and so the electrons are having to fight incredibly hard to get out of the center of the
atom Platinum I won't ask anyone to guess because I know everyone's feeling very nervous tonight is 72,000 EV so
again Platinum not that much bigger than but pulling those electrons even harder so it becomes very hard to escape but
the great thing about these being different energies is they're characteristic if you shoot a bunch of
different colored X-rays at things you can tell exactly what elements are there just by the energy that they
absorb so that's why we have free electron lasers and synchrotrons and R
there those are the energies that you're able to those are the energies you need
to identify if that element is present and different beam lines and different facilities all across the globe all have
different uh energies of x-rays that you do so the way you actually use the instrument is you write these proposals
you mail it off and they say we'll give you seven days in two years prepare really well because that's the only shot
you have uh can be very alarming but it's really amazing the information that
you can get from these facilities so what this looks like in practice is you have your X-ray light
and it goes in and it hits a sample this it as a powder the X-ray either transmits through the sample or you
measure it some other way and you really just see x-rays before you hit the sample and x-rays after you hit the
sample and you just subtract those and so if you have a ruum you expose it and
you see this peak remember this is my camera this is the X-ray camera I'm taking a photo of
it this is what my uh ruini looks like before it has a piece of light so before
it loses an electron so you photo excite it with your visible light pulse emulating the
sun nice lightning bolt on the renum and you can answer this question of has light created an electron and you're
going to answer that by if this peak moves left or right that Peak is going to change and if it changes you can say
hey the electron has left home lets you know no troll at the renum center you
can then go to a different beam line with a different amount of energy and you can look look at the Platinum same
exact thing you shine light on it start the electron leaving the renum
and if you see a change you can answer the question has the electron arrived on the Catalyst just by the movement in
this peak of this energy and again we're taking these x-ray photos incredibly fast using these lasers so your distance
and time can be fto or Pico so again every millionth of a billionth of a second you can take a picture take a
picture take a picture and so you can not only see has something arrived there how long did it take to arrive
there lastly I'm going to talk about nitrogen so nitrogen you can see much
lower energy but there's a lot more nitrogens throughout this molecule so if you shine light on it
start an electron moving you can identify exactly where on the molecule is the electron just by looking at one
of theen so if it's still around the ruini you're going to get a change in this
yellowish Peak if it's gone to the center of the bridge you're going to get a change on the greenish peak in the center if it uh is on the Platinum the
Catalyst part of your Bridge you're going to see a change in the highest energy Peak that blue one and this can
be incredibly diagnostic because now you're able to map the entire molecule and I uh with using one energy and
you're crucially able to look at the bridge step so why I mean you notice that you
might have noticed that the ranium had one Peak and the Platinum had one Peak but the nitrogen has three Peaks this is
because there's different chemical signatures you're measuring that in of the nitrogen so this is the where you're
moving an electron from the most core of the nitrogen to
the but this is a nitrogen atom it's just sitting in gas not interacting with anything when you put it into a molecule
it has a bunch of other atoms around it those other atoms are going to fill the veence shell so they're going to share
some electrons and the center of the nitrogen all the other electrons are going to respond to the presence of the
new ones they're going to pull in closer to the nucleus so kind of reacting back and forth what is around it and that's
going to what it's around it is going to change so the veence the core is much
closer the veence shell is full so it's having to go even further to escape this electron and just like we showed
earlier if the electron has further to go and it's a harder go job you have to
use more gas to get there and that's going to move your Peak and that's going to be diagnostic of what the nitrogen is
bound to so in this way we can start answering this question of what part of
the bridge is the electron getting stuck at if it's in the center of the bridge
you're going to see a change on the yellow and green Peak if it gets stuck on the ruum part of the bridge you'll
only see a change in the yellow Peak if it gets all the way to the Platinum part of the bridge and you see a change in
every single Peak you can then use that as traffic cameras and speed not speed
map do isn't word the speed how long it took to get somewhere um and that's incredibly
powerful so oh thought I already did that so let me talk a little about bit
about the actual experiment we're doing so this is a brand new inst station at
uh LCL so some of you might or might not know lcls is undergo an upgrade right now
we're becoming lcls 2 and what that means is we're super cooling an entire
portion of the accelerator and by super cooling it we can make our electrons multiply even more so the source is
going to be a million times brighter and once again you can imagine what that's going to do to your signal to noise if
you have a really bright light you can see better than a dim one like if you get a splinter you don't sit in your
living room you go into the bathroom under the brightest light you have to see as well as you possibly can so this
is going to really improve our signal to noise and so we built this new inst station called
kimri so this has a comedically complex sample delivery I say comedically
because you want to deliver these molecules to an x-ray beam but you don't want them to be a powder chemistry
doesn't happen in solid state it happens in solution interacting with the solvent in the environment you're mixing a bunch
of things together however uh sorry however nitrogen
absorbs at 400 EV I mentioned that a few times and that's because that's significant as soon as you go below
around a thousand EV of light energy you have these photons they're getting pretty weak and they're just going to
start interacting with everything including the atmosphere air any solvent you put it in any Optics so you have to
do the entire experiment under a vacuum chamber so this has to get to around 10
the minus 5 T that's you know space is something like 10us 12 to 10us 17 so not
quite at Space level but it's not very much air in there you have to use a
couple of different vacuum pumps to get it out there all right so do the entire experiment vacuum chamber people do that
but remember I said this is a liquid that I'm putting in so I'm shooting a liquid into a vacuum chamber and it
interacts with air so it's definitely going to interact with any sort of cell that I put this liquid in so going to
just shoot bare liquid I'm just going to Jet liquid into a vacuum chamber an exposed nozzle and you know I also I
don't one of the ways we do this is you sit a nice round jet people people know
how to make round Jets that's very reasonable and very nice but for x-ray
absorption you want a nice homogeneous surface so you want a really flat jet so
you want an ultra thin Jet and by ultra thin I mean you know a few Micron
thick so we designed this insane microfluidic chip design so instead of
jetting a round jet of water you have this micro chip and it has different channels and as the channels come in
they shoot liquid out the liquid hits into each other and splashes out in this Leaf form and then splashes out into a
leaf form and then splashes out into a leaf form and you can engineer these so delicately that you get something that's
maybe a millimeter wide and then only a micron
thick so over on the left is one of the first examples of this you had this kind of colliding chip and you can see there
was still a lot of interference it wasn't perfectly smooth I show that picture to show this converging nozzle
look how amazingly smooth that is you know that's smooth because you see those bands of light we actually use those
bands of light to figure out how thick this sample is and by the bands of light interacting you can figure out how thick
it is so again very thick this jet that this is a photo of is around five microns thick once again compared to
human hair that's 20 to 180 Micron thick so this is significantly thinner than
even the finest baby hair and it's a millimeter wide and we're shooting it into an exposed vacuum it's pretty
insane and you know we have one more problem this isn't reasonable at all
because these samples are precious they're so precious that just a few
grams of it is going to take a collaborator six months to years to synthesize you do not make friends if
you're like hey I want to do an experiment can I have like three years of your life making this one sample please and you know so we're like okay
we really want to do these measurements really precious samples you're going to synthesize those in like tens of
milligram amounts very very small and so you really want to ask someone for under a gram and so in order to do this we're
like great we're already shooting an insane liquid jet into a vacuum chamber let's also add this catcher so this is a
heated copper tube and it's like attached to its own vacuum chamber so you shoot a liquid jet exposed into
vacuum catch the water suck it out and recirculate the entire thing and so we only recirculate around 50 milliliters
of it that's significantly less than I have in this class um and this is just
it's so much engineering went into this I put the publication on there I know you're all going to rush out and read it
um it's very impressive because now instead asking for 16 or 20 grams of a
sample you can reduce that and ask for 500 milligrams which makes you lots of friends and has people actually want to
do an experiment with you um I went over this just
because I think this is one of the coolest things about science is the truly comedic things you do to make an
experiment work uh again 50 milliliters for around eight hours constantly being exposed to
things so we did this experiment last year and
we just did the first half of this bridge because this was a brand new inst station and again it was actually designed for LS2 when we have a million
times better Source but we went ahead and had to test it before we had that because of course just like do your
experiment with a million times less photons that's fine so we've did this experiment and we were able to show that
the electron does after being excited by light land on the bridge
however from preliminary results we're finding that uh there's also a troll
there the electron never wants to leave that bridge it's too stable it's too happy there's like a cafe on the bridge
and all kinds of other stuff it never actually wants to go all the way to platinum who wants to drive that far but the really important conclusion from
this isn't that we found one molecule that didn't work is that we found a mechanism for why it didn't work and we
built this system that's modular so the reason I have these drawn
in separate parts is because the entire ethos of this project is is to build these like building block linkers so
imagine these are Lego sets where you have a bunch of different electronic sceps and one Catalyst you pick the
electronic scepter that absorbs the color of light that you currently have or you have an electronic septer so
thing that turns sunlight into electrons and it's incredibly good at absorbing blue sunlight which hits your office at
the perfect angle each day to be very intense and so you can just swap out the electron acceptor the Catalyst and do
whatever chemistry you want using this Linker you've already designed so the example here if your Bridge doesn't work
like the ones we've used we can just substitute out a new bridge and again that still has nitrogens in it so this
technique I described is still going to be useful for figuring out if this system works so we've been presenting
this at conferences and scientific meetups recently every single person you
talk to is really excited about the ability to look at nitrogens this is because nitrogens are ubiquitous in
these types of systems here's a bunch of bridges you can notice that there's nitrogens everywhere nitrogen it's like
carbon it's one of the building blocks of all chemistry and it's incredibly important for understanding these
systems so so many people have come up to us and are really excited about when are you going to get the good enough
signal noise that you can move into harder solvents that you can move into this it's been really fun to talk to people about all of the different
experiments that you can do going forward and this is here at slack National Lab they've been building it the last few years it's going to be
really great so in conclusion kind of what I've talked about to you today is how you use
ultra short X-rays to track this electron movement in these kind of bridge system
in these light harvesting systems that are modular and this has a bunch of great applications uh it's going to be really
cool going forward so that is searching for trolls under the electron
Bridge
also always a plug for collaboration science as a team slack is a massive facility these are like 20 different
people who all work together just to do that one experiment and many many more again Christian Kunis catching light a
previous SLAC public lecture really great introduction to the chromophor so the light absorbing turning it into an
electron really great introduction on that highly recommend
that's all so uh Liz thank you very much I
guess there are some members of this team in the audience too want to raise your hand yeah team members over there
very good um so we have some time for questions so here's the deal with the
questions um this uh lecture is being recorded and it'll appear I guess in
about a week on YouTube on our SLAC YouTube channel if you'd like your question to be actually in the recording
which I hope you do uh please wait for the microphone to come to you and then talk into the microphone and then Liz
will answer the question so please raise your hand to ask a question and I'll recognize
you
sir hi thanks for the talk um I wanted to understand with the absorption lines
like like what what does that mean like a certain electron energy is being
absorbed and then you don't see it at the end or something or um do you mean
like from the solar Spectrum uh no no there was like the plots that you were showing to work out where the electron
had gone in that system yeah like this yeah yeah those ones yeah okay sorry can
you repeat your question just so sure yeah so what does that like peak mean does
that mean those electrons never reach the end or uh no this is the initial
Spectrum so this is at the ground state so before you ever photo excite it you're sending in this color of X-ray
light and it's going to based on just the identity of the
atom so an atom it exists at the center with the nucleus it's going to be positively charged and then you have
electrons existing in layers out of it is called a core level technique
x-rays are going to take one of the innermost electrons give it energy and leave and so that is the energy it takes
for an electron to leave the center of the atom and that energy the amount of
gas it takes and the distance it has to go that's going to change by everything that's around it so this is just a
ground state measurement and then if you photo excite it first these Peaks are going to move and you can identify what
part of the bridge it's on by which Peak moves oh but let's be a little could you go back to the
ranium the original Peak that you should yeah there we go so the question is why
is there a peak rather than a spread yeah and the yeah and oh um so it's a
bound transition versus ionization so ionization means the electron just leaves gets skyrocketed never sees home
again a bound transition means it's moving in between orbitals so it could be in between the closest Shell and the
one like third up or the closest Shell and the veilance and so it's a bound transition because it moves a specific
amount and then I don't show it here but each of these it has this pre Peak and
then there's kind of this big step function that happens after it and in atoms these levels are very discrete
they're definite energy so it's it's a very narrow band of x-rays that has this
large probability to go from exactly this shell to exactly that show and
that's used in all of these techniques and many other in the rest of chemistry too I should say and so Platinum is a
good example because you see that's sharp Peak and then you can already see the ledge and that's the ionization energy so it's a big shelf kind of with
a main Peak you thanks for the question uh who else
sir so I'm thinking about the exclusion PR ible and the fact that you can only have one electron with one quantum
number in one spot as these things move along the chain is it actually one electron going
each step or is one pushing on the end whole end the chain and pushing another
one out the other end can this technique actually just answer that question so the technique I think can't
actually answer that question because it's going to be a resolution issue and I think depending on the different system it's either going to be the same
electron because the electron it's moving in something called the lumo the lowest unoccupied orbital because
electron you know path of least resistance so it's hopping into the the lowest space and so the same electron is
moving because usually it's that one the idea is that the one electron has all this energy and so it's hopping it's
starting out it has been sent a bunch of energy and then you design it so that the bridge is lower is an easier path
for the electron than otherwise so it's usually the same electron that is just kind of bouncing around trying to find its lowest energy
place but I think like if you go into solid state materials they tend to think of it as like you know a phononic mode
of electrons bouncing against each other and things like
that yeah what made you decide to become a
scientist oh um I've always liked science and a lot
of stuff it's really fun but I had an internship as an engineer after my first
year of college and I hated it so much I changed my major I just yeah I worked in
a plant all summer and I just oh felt like I was selling happiness so like selling my soul
selling happiness for money I don't know how to phrase that and so I changed and a chemistry major and then at the end of
college I just really thought science was interesting thought I'd go see what grad school was
about thought it was
fun you me understand how you capture the data on the back end of these things
when you have ftoc resololution like what's the yeah what's the data
capture look that's coming out of this process so the data capture so it's ftoc
resolution but you measure the same step many many times and so the detectors only need to run out at like you know
per second or something like or per microsc something much much slower than that and so there's kind of three main
ways that you detect this this is a good slide to be on for that so you can either have transition mode which is
what I have documented here you just measure before and you put literally a CCD camera like a literal regular camera
that can detect afterwards or just a photo diode that's counting the number of
uh photons that are hitting it so that's one way the measurement that I actually have here is something called total
electron yield mode and so this is you grind up your sample into a powder and
put it onto conductive tape it's carbon tape that's sticky and you stick that onto a piece of uh copper something very
conductive and then you literally just pretty much attach a voltmeter and through the photoelectric effect a
photon hits this you create this electron and you measure the drain current that's just bleeding out of it
and that's called total electron yield mode so that's two different ways we can measure this the Third Way is
fluorescence so when shine a piece of light on it and generate this electron
that electron eventually loses its energy and if it loses it back to where it was it'll emit a characteristic uh
Photon and then you can put a photo out in front of it and so that's for when you have like a really cloudy sample or a solid or something like this so the
main three ways to detect it but luckily sample readout isn't a problem but that confused me so much when I started grad
school because I was like to seconds and what like our camera is reading out at half a second so yeah it's that you
do the same measurement many many times and then just by separating the distance
the the laser goes that gives you the ftoc resolution thank you can can I expand on
that question yeah please do how do you know how long it is between when the two laser pulses
hit uh so that is a really fun thing so you essentially measure something called
time zero and so it's a little bit harder in the X-ray but if you have two
Optical lasers you send one through a nonlinear material so you send an a
laser going this way you overlap another laser going this way and then you change the distance like a delay stage just add
a leg or something like that and through this material you actually will have it add photons together and it'll change
the momentum which is the angle so you have a crystal one laser goes this way one laser goes this way and when you
have time zero when they arrive at the same time a third laser comes out at an angle in between those and you stick a
white piece of paper behind your Crystal and you stare at it and stare at it and stare at it until you see a blue blip
and then you yell at your labmate go back go back go back and then you realign it and you just kind of manually move those so that calls time zero and
then again all light travels at about the same speed and especially the same speed
in a vacuum so literally by changing the distance of the path of the laser you're
changing the amount of time it takes to get there we literally we can literally have tape measures that will have like
millimeters on one side and Picoseconds on the other side and you can measure the distance because electron or light
moves at the same speed it's very fun it's very pretty please you have the next
question um I have two questions so the first is
um why do you only um push the electron from the innermost to the veence shell
and not just directly out of the at um so it's the type of measurement
I'm doing there's actually both kinds so you can have
um scattering so okay this is an absorption measurement so we are literally looking for that transition so
we're scanning standing across all these energies and seeing where it is because we're trying to identify the energy of
that veence band that's one of the things we're looking for so if you have a measurement where it kicks it out
that's going to be a lot of the scattering techniques which isn't what I do but basically they kick out that electron and then they track that
electron hitting other atoms and bouncing around and they see the energy of the electron after it's hit a bunch
of things so that is also an incredibly important technique uh um and then the
second is I know you showed a lot of the bridges where like nitrogen B because um
It's relatively versatile like carbon is there any other element that could achieve the same effect or that you've
experimented with so I haven't looked at a lot of uh other Bridges just because we're working on this nitrogen technique
but yes it's a huge field you actually have people who are even using like carbon Nanotubes or something like that
or yeah lots of different kinds of bridges um couple of the ones I show actually
have like zinc and carbons and stuff like that so there's so many different kinds of bridges and there's it's a huge
field of research uh let's see Fluorine and sulfur shows up a lot thank
you the
back um what what if you switched to heavy hydrogen duum would you expect any
new phenomenon from that I don't think I know what a heavy
hydrogen material is duum oh dyum yes you would expect a change from
that so if you do duum instead of water you're going to again be changing the
electron orbitals um I don't think I have a more specific
answer than that yeah it's duum in the um hydrogen generation process you want
to generate D2 instead of H2
it's just there's a lot more hydrogen so yeah there's a lot more hydrogen so duum and heavy atoms are really important
actually for this entire field of research and that is because this is chemistry so if it's hydrogen Evolution
you can deuterate your solvent the other parts of the Catalyst and you can actually use that to find out where did
the hydrogen that I'm using to do this reaction where did it come from am I harvesting hydrogen from the solvent is
it from like electron donor is it being ripped off of the like glass container
I'm making the experiment in you can deuterate various things and really track those to see what is contributing
the important part to the chemistry but for cars um we we just
don't it's expensive to collect enough dyum to power a car so you better do it
with ordinary hydrogen yeah plus slack narium is a big no no substance okay you
get a bunch of are really mean emails if they think you've lost a bottle of dyum it's very confusing especially if you
haven't lost a bottle of duum and you don't know what they're talking about okay um let's take two more questions
who has them uh
please so you mentioned uh lcs2 is that the right um and I think
there are two orders of magnitude higher Fidelity measurements that comes with that what what does that mean for your
experiments what are you going to be able to see uh that's going to be amazing for these experiments because
the sample right now we have to do it in water the reason you have to do this delivery in water to look at nitrogen is
because carbon absorbs at 250 EV and like that gentleman asked about there is
a shelf you're going to ionize your all of your solvent so it's going to make massive background and so uh if you have
a carbon solvent there's currently too big a background for you to be able to see your sample once you have these this
huge order of magnitude Improvement in your signal to noise you're going to be able to expand this experiment to
different solvents which is what it was designed for and you're going to be able to still have great signal to noise in
that and you're also of course going to be able to reduce the sample as we did this experiment at 100 Millar which
means that it's basically sludge and jetting sludge is like a huge problem I broke several pumps and probably made
enemies so yeah it's going to be really amazing said the inst was designed for the upgrade so it started receiving that
light maybe November and so they're really excited I think the first experiment is this February with the
upgraded light source so it's worth saying a little about this it's not that
the light is going to be a million times brighter it's that
you're going to have a million times more pulses per second of about the same size so the way that's achieved is by
using a different kind of accelerator than they're using now at slack the old
slack is based on a 1960s basically version copper accelerator they've just
cleared out a third of the tunnel at slack and put in an accelerator based on
superconductivity so you can bring that down to uh a degree or two above absolute zero and then this material is
um just it maintains High field and you don't lose power and you can shoot it
much more often and the idea is to shoot at a million pulses a second instead of
120 pulses a second for the ordinary slack and so um each each pulse can give
you data and so it's just G to be much more powerful it's gonna be amazing
we're gonna go from collecting something like a terabyte of data every few minutes to a terabyte of data per second
and yeah uh yeah I think there's a lot of concerns about how are we even going to
store that data and then I think we don't know how to do it right now and so like one example is there's someone
working on machine learning to look at all the pulses in real time and just be like never mind that's not real don't
even store that that's not real data well you know what I'm a particle physicist and we deal with this at the LHC already so yeah um in principle we
know how to do it and it's just bringing those techniques to this field uh which we hope is ready for it another really
cool thing about the upgrade that I'm excited about is uh x-ray sources like this these XLS the X-ray free electron
lasers there's six of them in the world they have this problem that that's called J and that's because this source
is so stochastic that your X-ray it's might not arrive exactly where you clock time zero it's going to be time zero
plus or minus a Picosecond and that Picosecond can be very frustrating if
you're looking for like a one PC Dynamic so you actually have to have a separate entire experiment that has to happen
during yours where you just clock when did the erex arrive and then you have to do a bunch of complicated math to like
Resort all of your data and something about super cooling the accelerator I understand I keep asking people is
something about this it's going to have significantly less Jitter something on the order of 20 ftto seconds rather than
900 ftto seconds and so that's going to simplify data processing and simplified
data collection for in time because again these are kind of high stress experiments where it's like 5 a and you
have a 12-hour shift and you're just like ah make this Choice make this Choice do we move the time or not so
it's I'm really excited about that it sounds cool okay anyone want to ask the last
[Music]
question please I may have missed this but I was
wondering how do you actually build the bridge is it just a solvent and you spr the lasers through them or can you
explain that a bit more how do you build the bridge so that's going to be a synthetic chemist and so uh these Bridge
so I have the molecule up here now so you basically take parts of the
bridge and then you sometimes expose them to light and it causes bonds to form I'm not a synthetic chemist I don't
know how to explain this well really impressive people mix things
together in weird temperatures with weird gasses and
great final question in your organic chemistry course in college you get um much
simpler problems like that assembling different bonds to get what you want but the complicated ones it's just black
magic yeah and it's really impressive people have gotten the Nobel Prize for
uh introducing new kinds of black magic that enable you to do previously inaccessible syntheses and so now we're
profiting from all of that to actually get these bridges in the first place yeah and that's actually one of the
coolest things about these donor Bridge acceptor applications is for this uh the area of photochemistry so literally
using light as a reagent in your chemical reaction is a booming field right now in the last 10 years there's
started to be lots of Publications on it and pharmaceutical companies actually doing photochemistry as like inline
industrial processes basically uh light can be a very targeted reagent so it can
do something like helping you with the handedness of a molecule so handedness is called chirality in chemistry and so
that's going to be take an orange and a lemon the thing you're smelling literally the same molecule just
different handedness they're mirror reflections of each other and another example of that is a lot of medicines
are amazing and if you have the wrong handedness that turns the wrong way that's actually going to be a poison there's something called the thalidomide
Scandal where in France in the seven 7s maybe the 80s a bunch of pregnant women
huh 60s great so okay y'all know about the fomite Scandal for those of you who
don't basically one-handedness of the molecule is a treatment for morning
sickness and so a lot of French women were given this to treat morning sickness and it caused the other
handedness of the molecule causes extreme birth defects and so there's a generation of the lamide babies who are
born with these extreme birth defects and plug for women's scientists uh was in and it was approved throughout Europe
but the FDA it was not approved for use in America which is why a lot of Americans don't know about it and it was
a single woman at the FDA who had a chemistry background and was like the research on this is not good this we
should not be giving this to pregnant women and she had a lot of political pressure from her boss and a like bosses
being like no no just pass it it's fine everyone else said it's fine and so just an example of one person thinking
something through carefully can really change and yeah got to be very conscientious and careful in science but
uh point of that story was that light can be a very targeted reagent and so it can help you synthesize just one-h
handedness of a molecule rather than the others just because those are going to be slightly slightly different energies and so maybe synthesizing at 455
nanometer versus 465 nanometer light which is both blue but it'll have a slight difference in the outcome of that
reaction thank you well let's thank Liz again this has been
very okay um I apologize that uh this
Auditorium is going to go out of commission during March so we will not have the traditional March public
lecture but we will have one at the end of May it'll be pretty fascinating and every two months we'll bring up another
one of these if you want to get on our mailing list uh go to the slack website look for public lectures and there will
be a way to get on the mailing list for these events and we hope to see you in May and in the future so thanks to you
all for coming and I hope you've had a good
time