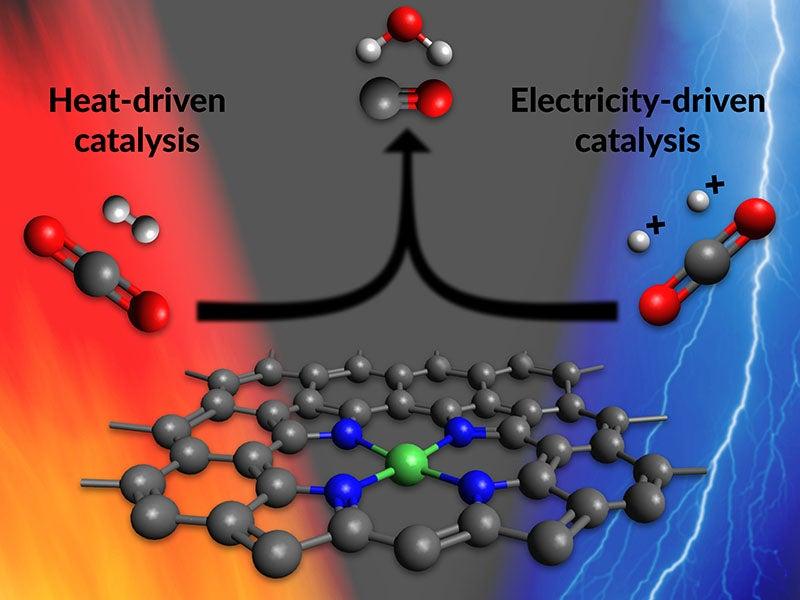
Scientists show a single catalyst can perform the first step of turning CO2 into fuel in two very different ways
Their work aims to bridge two approaches to driving the reaction – one powered by heat, the other by electricity – with the goal of discovering more efficient and sustainable ways to convert carbon dioxide into useful products.
By Glennda Chui
Virtually all chemical and fuel production relies on catalysts, which accelerate chemical reactions without being consumed in the process. Most of these reactions take place in huge reactor vessels and may require high temperatures and pressures.
Scientists have been working on alternative ways to drive these reactions with electricity, rather than heat. This could potentially allow cheap, efficient, distributed manufacturing powered by renewable sources of electricity.
But researchers who specialize in these two approaches – heat versus electricity – tend to work independently, developing different types of catalysts tailored to their specific reaction environments.
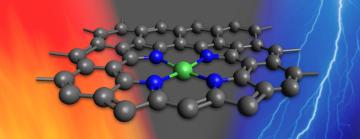
A new line of research aims to change that. Scientists at Stanford University and the Department of Energy’s SLAC National Accelerator Laboratory reported today that they have made a new catalyst that works with either heat or electricity. Based on nickel atoms, the catalyst accelerates a reaction for turning carbon dioxide into carbon monoxide – the first step in making fuels and useful chemicals from CO2.
The results represent an important step toward unifying the understanding of catalytic reactions in these two very different conditions with distinct driving forces at play, said Thomas Jaramillo, professor at SLAC and Stanford and director of the SUNCAT Institute for Interface Science and Catalysis, where the research took place.
“This is a rarity in our field,” he said. “The fact that we could bring it together in one framework to look at the same material is what makes this work special, and it opens up a whole new avenue to look at catalysts in a much broader way.”
The results also explain how the new catalyst drives this key reaction faster when used in an electrochemical reactor, the research team said. Their report appeared in the print edition of Angewandte Chemie this week.
Toward a sustainable chemistry future
Finding ways to transform CO2 into chemicals, fuels, and other products, from methanol to plastics and synthetic natural gas, is a major focus of SUNCAT research. If done on a large scale using renewable energy, it could create market incentives for recycling the greenhouse gas. This will require a new generation of catalysts and processes to carry out these transformations cheaply and efficiently on an industrial scale – and making those discoveries will require new ideas.
In search of some new directions, SUNCAT formed a team of PhD students involving three research groups in the chemical engineering department at Stanford: Sindhu Nathan from Professor Stacey Bent’s group, whose research focuses on heat-driven catalytic reactions, and David Koshy, who is co-advised by Jaramillo and Professor Zhenan Bao and has been focusing on electrochemical reactions.
Nathan’s work has been aimed at understanding heat-driven catalytic reactions at a fundamental, atomic level.
“Heat-driven reactions are what’s commonly used in industry now,” she said. “And for some reactions, a heat-driven process would be challenging to implement because it may require very high temperatures and pressures to get the desired reaction to proceed.”
Driving reactions with electricity could make some transformations more efficient, Koshy said, “because you don’t have to heat things up, and you can also make reactors and other components smaller, cheaper and more modular – plus it’s a good way to take advantage of renewable resources.”
Scientists who study these two types of reactions work in parallel and rarely interact, so they don’t have many opportunities to gain insights from each other that might help them design more effective catalysts.
But if the two camps could work on the same catalyst, it would establish a basis for unifying their understanding of reaction mechanisms in both environments, Jaramillo said. “We had theoretical reasons to think that the same catalyst would work in both sets of reaction conditions,” he said, “but this idea had not been tested.”
A new avenue for catalyst discovery
For their experiments, the team chose a catalyst Koshy recently synthesized called NiPACN. The active parts of the catalyst – the places where it grabs passing molecules, gets them to react and releases the products – consist of individual nickel atoms bonded to nitrogen atoms that are scattered throughout the carbon material. Koshy’s research had already determined that NiPACN can drive certain electrochemical reactions with high efficiency. Could it do the same under thermal conditions?
To answer this question, the team took the powdered catalyst to SLAC’s Stanford Synchrotron Radiation Lightsource (SSRL). They worked with Distinguished Staff Scientist Simon Bare to develop a tiny reactor where the catalyst could expedite a reaction between hydrogen and carbon dioxide at high temperatures and pressure. The setup allowed them to shine X-rays into the reaction through a window and watch the reaction proceed.
In particular, they wanted to see if the harsh conditions inside the reactor changed the catalyst as it facilitated the reaction between hydrogen and CO2.
“People might say, how do you know the atomic structure didn’t change, making this a slightly different catalyst than the one we had previously tested in electrochemical reactions?” Koshy said. “We had to show that the nickel reaction centers still look the same when the reaction is finished.”
That’s exactly what they found when they examined the catalyst in atomic detail before and after the reaction with X-rays and transmission electron microscopy.
Going forward, the research team wrote, studies like this one will be essential for unifying the study of catalytic phenomena across reaction environments, which will ultimately bolster efforts to discover new catalysts for transforming the fuel and chemical industries.
Parts of this study were carried out at the Stanford Nano Shared Facilities, the Canadian Center for Electron Microscopy and the Center for Nanophase Materials Sciences (CNMS) at DOE’s Oak Ridge National Laboratory. CNMS and SSRL are DOE Office of Science user facilities. Major funding came from the DOE Office of Science, including support from the Joint Center for Artificial Photosynthesis, a DOE Energy Innovation Hub.
Citation: David M. Koshy et al., Angewandte Chemie, 06 April 2021 (10.1002/anie.202101326)
For questions or comments, contact the SLAC Office of Communications at communications@slac.stanford.edu.
SLAC is a vibrant multiprogram laboratory that explores how the universe works at the biggest, smallest and fastest scales and invents powerful tools used by scientists around the globe. With research spanning particle physics, astrophysics and cosmology, materials, chemistry, bio- and energy sciences and scientific computing, we help solve real-world problems and advance the interests of the nation.
SLAC is operated by Stanford University for the U.S. Department of Energy’s Office of Science. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time.